Want to understand the unique workings of an absorption chiller?
This one article is enough – let’s dive into its fascinating working principle. Absorption chillers are remarkable machines that use a heat source to provide cooling. But what truly sets these systems apart are the fundamental principles they apply.
Table of Contents
Here are seven key principles underpinning this clever cooling technology:
- 1.Liquids boil and become gases upon heating, while gases condense into liquids when cooled.
- 2.A liquid’s boiling point drops when the pressure above it is reduced.
- 3.Heat travels naturally from warmer to cooler areas.
- 4.A higher concentration of a solution increases its ability to attract and absorb particular gases.
- 5.The boiling point of a solution can change based on the solute’s nature and the solution’s properties.
- 6.The perfect pair of refrigerant and absorbent is crucial; this duo must have a strong chemical affinity, meaning they combine well and enhance the overall efficiency of the cooling cycle.
- 7.The system’s efficiency hinges on the absorbent’s regeneration—it must continuously be re-concentrated to maintain its absorbing capabilities.
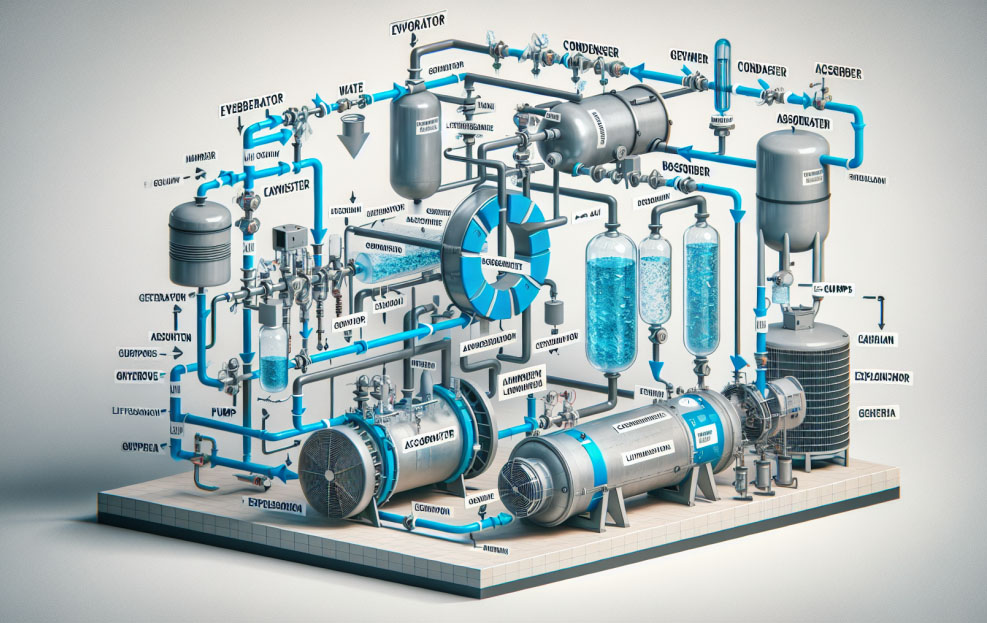
Now, let’s embark on a step-by-step journey detailing the seven stages of the absorption chilling cycle:
- STEP 1: Pressure DependenceUnder a high-vacuum environment, water’s boiling point dramatically decreases. Even at a reduced temperature of 3.7 degrees Celsius (38.66 degrees Fahrenheit), water can start to boil when the pressure drops to just 6 mm-Hg (absolute).
- STEP 2: The Absorbing Nature of Lithium Bromide (LiBr) possesses a natural ability to absorb water thanks to its chemical affinity. The level of absorption is a balancing act—greater when the concentration is high and the temperature is low.
- STEP 3: Regenerating the AbsorbentTo continue absorbing, diluted LiBr must be re-concentrated. This is achieved by applying a heat source, which could come in various forms like steam, flue gases, hot water, or even direct fuel firing.
- STEP 4: Refrigerant Release and RecoveryAs the absorbent is heated, the previously absorbed refrigerant is released as vapor. This vapor then migrates to a cooler chamber where it condenses back into its liquid form, thereby getting ready for the next cycle.
- STEP 5: Creating a VacuumCreating a vacuum in the evaporator section reduces the boiling point of the refrigerant, allowing it to vaporize and absorb heat from the surroundings, thus creating the cooling effect.
- STEP 6: Heat Exchange EfficiencyThe heat from the refrigerant vapor is transferred to the cooling water in the condenser, maximizing the chiller’s efficiency by ensuring the refrigerant loses its heat and returns to a liquid state expediently.
- STEP 7: The Thermochemical CompressorCompleting the loop, the thermochemical “compressor” constituted by the affinity between absorbent and refrigerant enables the refrigerant to evaporate at low pressures and temperatures, repeating the cycle of absorption and thereby sustaining the cooling process.
To summarise
By navigating through these seven basic principles and procedural steps, you can now appreciate the elegance and complexity of absorption chillers. From a scientific marvel to a practical necessity, these chillers not only cool our spaces but also marvelously exhibit the power of thermodynamics at work.
Contact Us
Shandong Hongrui Co. is a leading supplier of lithium bromide. Whether you need lithium bromide for air conditioning systems or other applications, we can help. Contact us now to learn more about lithium bromide products. Our sales team will quickly provide you with a quote and reduce your costs.